N Each Period of the Periodic Table, the ______________ Family Member Is the Largest Atom.
![]()
The periodic table is organized by electron configuration in atoms. You'll get the almost out of this section if you study the pages on electrons and atoms.
Organizing the elements
Now that we've seen how electrons are bound to atoms, that they occupy orbitals with strict rules about how many atoms occupy what kind of odd-looking region effectually the nucleus, we're in a position to understand the layout of the periodic table. That agreement will help u.s.a. to use the tabular array equally a tool for solving all kinds of problems, and for remembering facts that are piece of cake to forget - the table volition go far like shooting fish in a barrel.
Kickoff permit's have a expect at the periodic table. Click on the one below to print it. I'll hash out some of its features below using smaller diagrams and pulled-out sections, and then yous might desire to refer to the full table often.

The periodic tabular array is the key to unlocking the secrets of many chemical problems. Proceed 1 close and use it.
The first thing that probably strikes you about the table is its odd shape. You lot've seen it before, merely y'all've probably never understood why it's laid out like it is. It'south all about the electron configurations. Have a look at this table, in which each element has been replaced by its electron configuration, to sympathize:
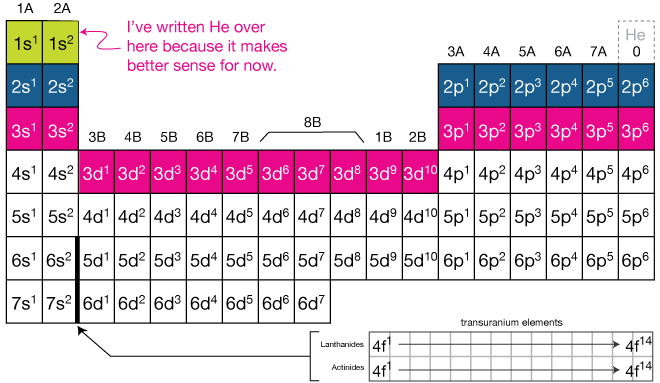
Await at the showtime (light-green) row in the table higher up. Hydrogen and Helium are elements one and 2, respectively, and have those numbers of electron (we always assume neutral atoms in the table). The electron configurations are H: 1s1 and He: 1s2. Afterward that, the n=i shell is total; it contains but an south-orbital and that can only hold two electrons. I've placed He over next to H in this table just to show that it lines up with all of the elements below information technology. We generally place information technology on the far right (like the starting time table) for another reason (I'll get to that later).
At present the blueish row: Hither the north=2 row is being filled. Start the s-orbital is filled with two electrons (Li and Be), then across to the six p-orbital electrons, ending with Neon,
which has a full northward=2 shell - no more than electrons will fit into the n=ii shell. Discover that He, Ne and all of the atoms below Ne accept full shells with increasing northward. These atoms also happen to be the to the lowest degree reactive atoms in the tabular array - nutrient for idea.
Now the n=three shell (
Organization of the periodic table
The periodic table is bundled in gild of orbital filling, according to the diagonal dominion. The first two columns fill up due south-orbitals. The rightmost six columns fill p-orbitals. The middle group of ten fills the d-orbitals, and the Lanthanide and Actinide series (cake below the main table) fill the f-orbitals.
The periodic tabular array is merely another expression of the diagonal rule of electron-orbital filling, and can exist used to write the electron configuration of any atom, just by reading left-to-correct, top-to-bottom.
Now we can address the issue of the relative stability of atoms. Information technology was already noted that elements in the rightmost column of the tabular array are specially non-reactive. In fact, their electrons are tightly jump and difficult to remove. Annotation that all of these atoms have eight electrons in their outermost (highest north) beat out.
The octet rule
It is difficult in this department non to personify atoms, but it helps: Oftentimes it is said that atoms "desire to have" eight electrons in their outer crush - the octet rule.
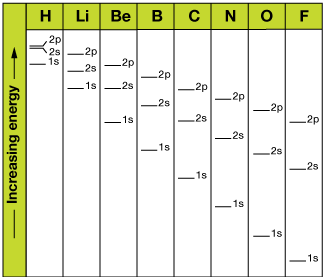
Nature e'er seeks the land of lowest energy. It'due south why a ball rolls downhill spontaneously, only never up. At right are the 1s, 2s and 2p orbital energies of a few atoms in the offset and second rows of the tabular array. Detect that as nosotros add electrons the energies decrease (but remain in the aforementioned social club). By the fourth dimension we become to Neon, with 8 electrons in the north=two shell, the cantlet so stable it is inert (doesn't react).
Another way of expressing the octet rule is to say that an atom tends to lose or gain electrons in club to have eight electrons in its outer trounce, or total south- and p-orbitals. Note that the periodic table (and the diagonal rule) prescribe that the p-orbitals of any level are filled earlier its d-orbitals are filled.
The inert gases
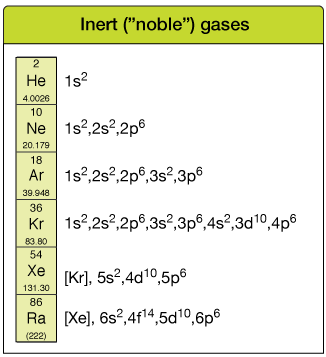
The elements in the rightmost column of the tabular array are called the inert gases. They all be every bit gases at room temperature and they all take total outer-shell octets, which makes them unreactive (inert).
Columns in the periodic table are chosen groups, and rows are chosen periods. Elements in groups tend to accept similar chemical properties (e.g. all gases, all inert).
Note that when the electron configuration gets cumbersome, it's OK to abbreviate the "core" configuration using another inert gas symbol, usually in [ ].
At present lets explore some of the other groups.
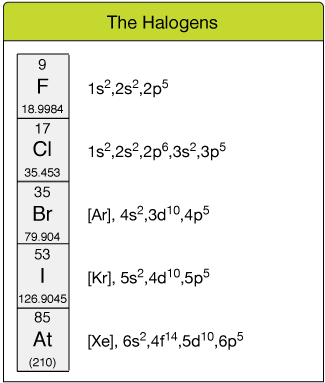
The halogens
The 2d grouping from the correct contains the Element of group vii group. These atoms all take seven electrons in their outer shells, ane curt of a full octet and a much lower energy.
Reactive -1 anions
As a issue, these atoms tend to "steal" electrons from other atoms; they have a loftier electron affinity ("similar" for electrons). Thus, the halogen atoms tend to exist as -1 ions, F-, Cl-, Br-, I- and At-.
Now we're getting somewhere. We've used electron configuration and its manifestation in the periodic table to find a whole group of atoms that be mainly equally -one ions.
On to another interesting and important groups. ...
The chalcogens

The elements in the oxygen cavalcade are sometimes (not often) referred to equally the chalcogens. They are normally simply referred to as the oxygen group. They lack 2 electrons to accept a total octet, so they tend to form -2 ions past picking upwards more weakly leap electrons from surrounding atoms.
-2 anions
These atoms tend to exist less as ions, however, and more as neutral atoms in molecules. At that place well be much more to say nigh this subsequently.
Physically, the chalcogens are a mixed group: O is a gas, Due south is a nonmetallic solid, and Se - Po are metals. Polonium is radioactive, and found every bit the source of ionizing radiation in many smoke detectors.
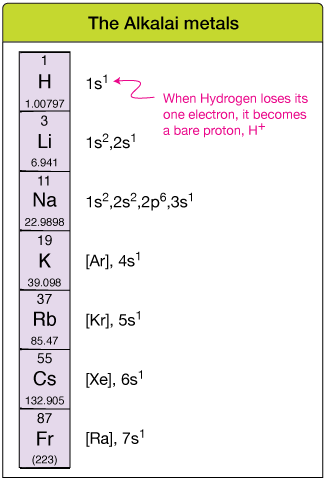
The Alkali Metals
The first column of the periodic tabular array (the first group) is the Element of group i group. This grouping contains the simplest element, Hydrogen.
The authentication of this group is that the electron configuration of each element is that of the inert gas just "behind" it in the tabular array, plus one electron in the adjacent higher s-orbital.
Reactive +1 cations
Because there is so much energetic stability to be gained by losing this electron (more properly, having it taken by an cantlet with more than of a demand for a spare electron), these atoms tend to lose one electron to go a +ane cation.
The Hydrogen cation, H+, plays a crucial role in many chemical systems. Note that Hydrogen contains merely a proton in its nucleus (except for its isotopes 2H, or deuterium, and 3H, or tritium). That means the Hydrogen cation is but a bare proton.
When yous meet an atom from the start group, you tin can be reasonably sure that information technology's a +1 ion in near any context.
Alkaline-World elements

The Alkaline Earth elements have two electrons more than the next lower inert gas, two more than than the most stable octet available, so they tend to lose two electrons to become more stable. Thus Alkaline metal Earths tend to exist mainly every bit +2 cations.
+two cations
In one sense, Helium belongs in this grouping. We classify it over with the inert gases considering it has a full n=1 shell, and considering it is quite inert. Helium is straight involved in only a few rare chemical reactions.
In the next few sections we'll have a look at periodic trends that can be gleaned from the tabular array. Often we can use the table to tell whether i element expresses more of some holding than some other merely from the relative positions of the two in the tabular array.
Chemical similarity
Elements within a grouping (cavalcade) of the periodic table tend to have very similar chemical properties. The properties of an element arise mainly from the outer-trounce electron configuration.
Other classifications
In the periodic tables below, elements have been colour coded to help y'all acquire well-nigh some of the classifications of atoms. We'll start with the so-chosen standard states of elements, the state (solid, liquid or gas) likely to exist institute at room temperature and standard atmospheric pressure.
Nearly elements are solids. The lightest and nigh stable are gases, like the inert gases. Just bromine and mercury (Hg) are liquids, although some of the solid elements melt at fairly low temperatures.
Metals, nonmetals, metalloids
The elements in this periodic table have been classified into six categories. Nigh elements have some kind of metallic graphic symbol, which we'll define afterwards. The metals in groups 1 and 2 tend to exist soft and very reactive (some explosive when mixed with h2o!).
The transition metals are some of the best conductors of rut and electricity. The metalloids, which lie between metals and nonmetals, are interesting and very of import because under the right weather, they can be coaxed to take metallic or nonmetallic properties. This makes them particularly of import in the electronics industry.

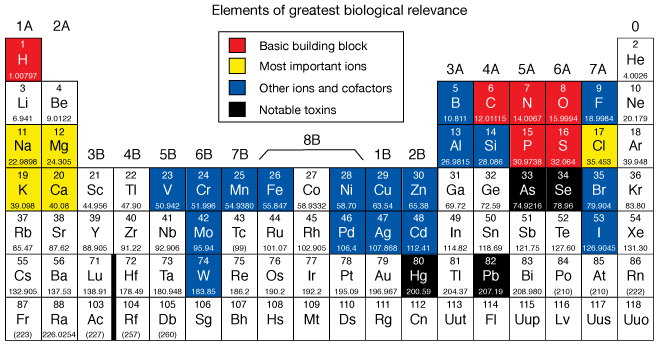
Biologically-relevant elements
A relative handful of elements are key components of biological systems on Earth. Carbon, Nitrogen, Oxygen, Phosphorus, Sulfur and Hydrogen are the backbone atoms of all proteins, nucleic acids, carbohydrates and lipids.
Ions similar Sodium and Magnesium are crucial electrolytes in and between cells, and many metals are essential cofactors of enzymes, without which enzymes can't work properly.
From where do elements come?
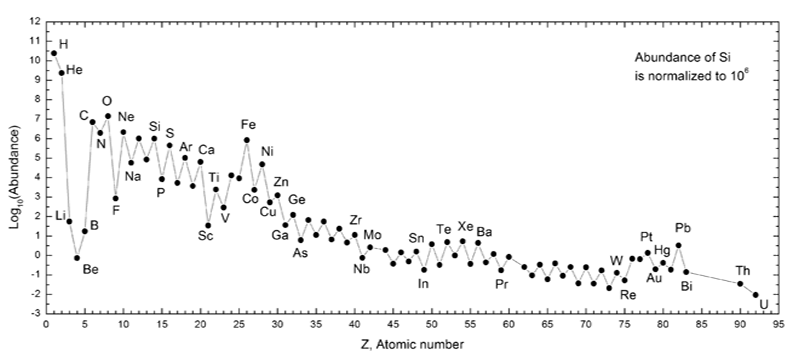
Cosmological theory tells us that at the beginning of the universe, the Large Bang if you lot volition, mostly H and He were created. Fluctuations in the density of the expanding gases caused some regions to coalesce and create more of an attractive force on the atoms effectually them - gravity. As these areas became increasingly dense, compressed and hot, stars were formed. In the intense heat and gravitational pressure of stars, smaller elements are fused to create larger ones. Our own sun fuses atoms upwards to Iron, but mostly fuses Hydrogen atoms into Helium.
Whatsoever atom in our solar arrangement larger than Iron, and probably quite a bit of what's here that's smaller,
had to come from other, hotter stars over the eons. Certain large elements can probably but be formed in the hottest stars, the supernovae.
Scientist and great science communicator, Carl Sagan, used to say that "we are all made of starstuff." All of the elements that compose us and our globe came from stars, some unimaginably far away.
Below is a graph of the distribution of relative abundances of the elements in our solar system. Note that information technology'southward on a log calibration, and so for example, in that location is roughly ten times (10ane) the corporeality of H as He.
In another department, we'll learn about other chemical trends that show up in the periodic table. We've only begun to get a experience for its usefulness. Proceed yours with you and await at it once in a while. Keep it nearby when you lot do issues. Sleep with it. Talk to it. In that location are likewise a number of bang-up periodic tabular array apps available for your electronic devices.
How much of the periodic tabular array should I memorize?

Your chemistry teacher might disagree with me, so be conscientious here, but my view is this:
I ask my students to memorize the showtime x elements of the table: H, He, Li, Be, B, C, N, O, F and Ne. These are the elements at the meridian of each column (grouping), and they are generally representative of the other elements in the group. If you know these elements, and then you've got a framework on which to build a memory of the other elements in the grouping as you report chemistry. Shortly yous'll assimilate a lot more elements into your long-term retentiveness and what gets stored there will make some logical sense.
For example, all of the noble gas elements (under He) are much more similar than different. Information technology'southward a more than productive way, in my view, to build a functional memory of the elements.
![]()
xaktly.com past Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created past me and I reserve all rights every bit to their utilize. Any opinions expressed on this website are entirely mine, and practice not necessarily reflect the views of whatsoever of my employers. Please feel gratis to send any questions or comments to jeff.cruzan@verizon.net.
Source: https://xaktly.com/Chemistry_PeriodicTable.html
0 Response to "N Each Period of the Periodic Table, the ______________ Family Member Is the Largest Atom."
Post a Comment